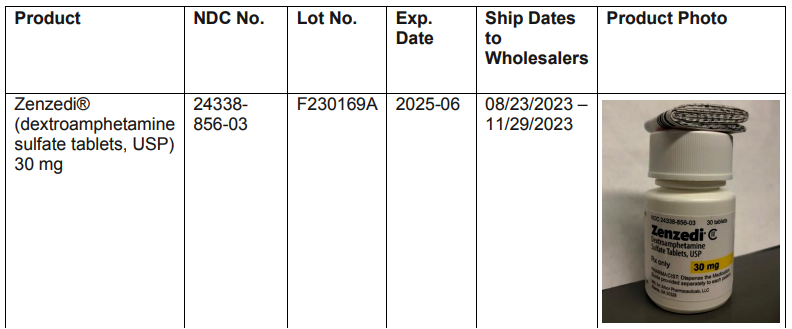
The Ministry of Health is advising of a voluntary recall notice for Zenzedi CII (dextroamphetamine sulfate tablets, USP) 30 mg.
The recall was issued by Azurity Pharmaceuticals, Inc. due to the drug Carbinoxamine Maleate, which was found in the packaging.
Zenzedi is a prescription medicine for the treatment of Narcolepsy (sleep disorder) as well as for Attention Deficit Hyperactivity Disorder (ADHD).
Patients who unknowingly consume Carbinoxamine Maleate may experience drowsiness, central nervous system depression, increased eye pressure, enlarged prostate urinary obstruction, and thyroid disorder.
For patients with ADHD and Narcolepsy, there is the possibility that accidents or injuries may occur due to the drug’s sedating effects.
The Ministry says this product is not registered for sale in the country, however, out of an abundance of caution, persons who may be in possession of the recalled products are being advised to discontinue use immediately and return the product to the place of purchase where possible.



Responses