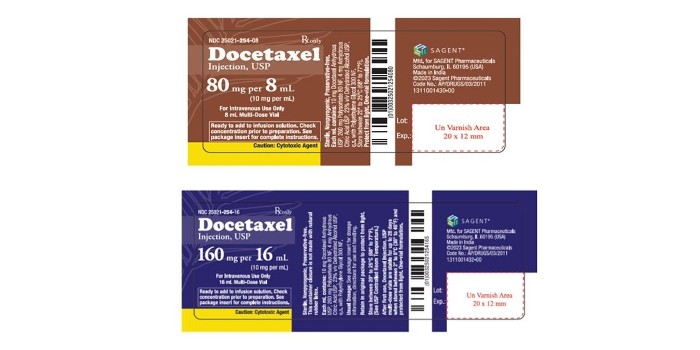
There is a voluntary recall of two lots of Docetaxel Injection, USP.
The Health Ministry says the international distributor, Sagent Pharmaceuticals, has recalled the product due to the potential presence of particulate matter from the stopper.
The recall is for the 160 mg per 16 mL multi-dose vials with the lot number F1030001 and the 80 mg per 8 mL multi-dose vials with the lot number F1040001.
The injection is indicated for the treatment of patients with certain types of cancers.
While the recalled products are not registered for use in T&T, out of an abundance of caution, the Health Ministry is advising persons who may be in possession to return the product to the place of purchase.


Responses