There is a voluntary recall of Digoxin Tablets USP, 0.125mg and one lot of Digoxin Tablets USP, 0.25mg.
This product is used for the treatment of mild to moderate heart failure.
The recall was issued by product manufacturer, Marlex Pharmaceuticals Incorporated, due to a label mix-up.
It says bottles of Digoxin Tablets USP, 0.125mg, were incorrectly labelled and contain Digoxin Tablets USP, 0.25mg.
Meanwhile, bottles of Digoxin Tablets USP, 0.25mg were incorrectly labelled and contain Digoxin Tablets USP, 0.125mg.
This can lead to either overdosing or underdosing, though the company says it not received any reports of adverse events related to the recall.
Marlex Pharmaceuticals Incorporated has identified the following lots for recall:
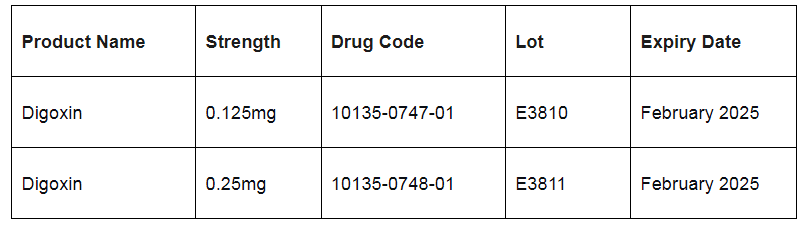
The Health Ministry says while the recalled products are not registered for sale in T&T, out of an abundance of caution, persons who may be in possession of the recalled product are advised to discontinue use and return the product to the pharmacy unit of the nearest Public Health Facility.



Responses